An average reaction rate is calculated as the change in the concentration of reactants or products over a period of time in the course of the reaction. An instantaneous reaction rate is the rate at a particular moment in the reaction and is usually determined graphically.
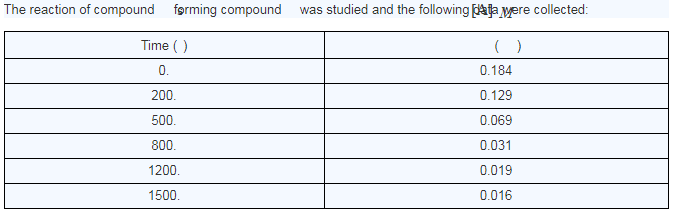
What is the average reaction rate between 0. and 1500. s?
rate = ??? (3 sig figs)
What is the average reaction rate between 200. and 1200.s ?
rate = ??? (3 sig figs)
What is the instantaneous rate of the reaction at t=800. s?
rate = ?? (2 sig figs)
Answer:
Average rate = (0.184-0.016 M) / 1500 s=1.12*10^-4
Average rate = (0.129 - 0.019 M) / 1000 s=1.1*10^-4
For the third one, you need to graph the points and determine the slope of the line at t=800 seconds.