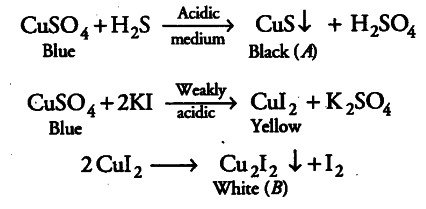
An aqueous blue coloured solution of transition metal sulphate reacts with $H _{ 2 }S$ in acidic medium to give a black precipitate ‘A’ which is insoluble in warm aqueous solution of KOH. The blue solution on treatment with KI in weakly acidic medium turns yellow and produces a white precipitate ‘B’. Identify the transition metal ion. Write the chemical reactions involved in the formation of ‘A’ and ‘B’.
A can be the black precipitate of CuS. B can be cuprous iodide which could have formed after the decomposition of cupric iodide.
(ii) Due to lanthanide contraction, the atomic radii of 4d and 5d transition series elements are almost same. That’s why, Zr (Z = 40) and Hf (Z = 72) have almost identical radii.