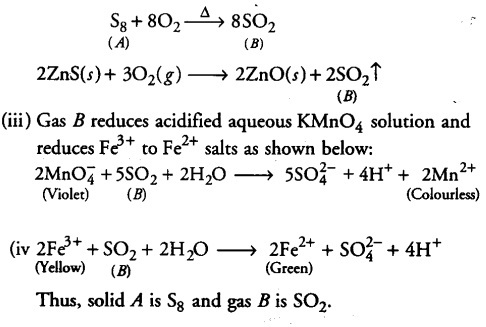An amorphous solid A burns in air to form a gas B which turns lime water milky. The gas is also produced as a byproduct during roasting of sulphide ore. This gas decolourises acidified aqueous KMn04 solution and reduces Fe3+ to Fe2+. Identify the solid ‘A’ and the gas ‘B ’ and write the reactions involved.
(i) Since, the byproduct of roasting of sulphide ore is S02. It also turns lime water milky. Therefore, gas ‘S’ must be S02.
the gas B is obtained when amorphous solid ‘A’ burns in therefore, amorphous solid ‘A’ must be sulphur, Sg