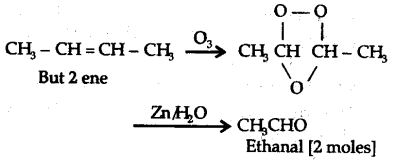
Alkene A contains 3C—C, 8C—H a bonds and one C—C \pi bond. Alkene A has one C = C double bond. An aldehyde containing one —CHO group and having molar mass of 44 a.m.u. has to be C${{H}{3}}—CHO and since two moles of C{{H}{3}}CHO are obtained by ozonolysis of alkene A, the alkene has to be joined by two C{{H}{3}}CH groups by a double bond. It has to be C{{H}{3}}—CH = CH—C{{H}_{3}}$, i.e., But 2 ene. But-2 -ene contains 3C—C a bonds, 8C—H a bonds and one C—C n bond.