Ammonia, NH3, is a weak base with a Kb value of 1.8x10^-5.
- What is the pH of a 0.390M ammonia solution?
- What is the percent ionization of ammonia at this concentration?
Concepts and reason
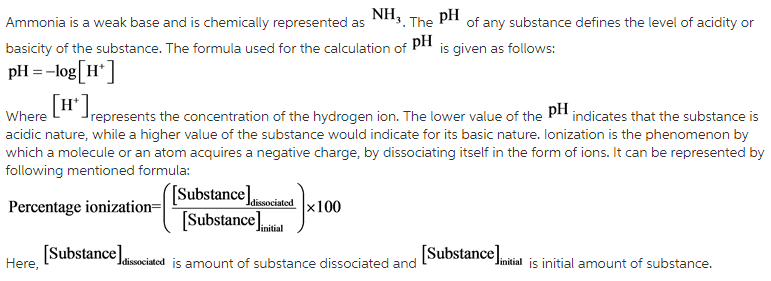
The concept of ![]() is used for solving this problem. The
is used for solving this problem. The ![]() (power of hydrogen) of any solution is calculated by measuring the negative logarithmic concentration of hydrogen ions in a solution. Furthermore, the percentage of ionization of any particular ion can be calculated using the unitary method, by finding out the number of ions dissociated from the initial concentration of the ions.
(power of hydrogen) of any solution is calculated by measuring the negative logarithmic concentration of hydrogen ions in a solution. Furthermore, the percentage of ionization of any particular ion can be calculated using the unitary method, by finding out the number of ions dissociated from the initial concentration of the ions.
Fundamentals
Answer:
Ammonia dissociates into ammonium and hydroxide ion when dissolved in water. The equation for the dissociation of ammonia can be represented in the following manner;
![]()
Furthermore, considering the concentration of ammonia that dissociates to be ‘x’, the initial and final concentration of the ammonia, ammonium ion and hydroxide ion can be represented as follows:

Explanation:
The molar concentration of the ammonium ion that has been supposed to be dissociated can be found out using the formula for dissociation constant, which has been mentioned below:
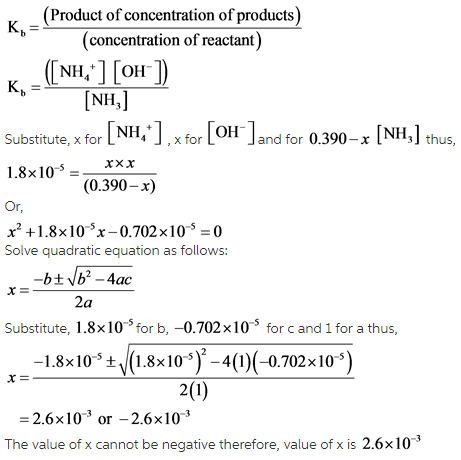
Explanation:
The concentration of hydrogen ion can be calculated using the formula mentioned below:
Explanation:
The percent ionization of the ammonium ion is calculated by estimating the fraction of ammonia dissociated from the initial concentration. The percentage ionization has been calculated as follows:
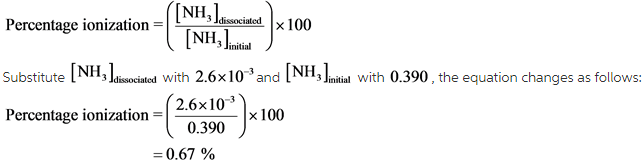
Explanation:
The PH of the solution can be estimated if the concentration of the hydrogen ion is known, for which the dissociation constant equation was needed to be employed. The percent ionization is given as the ratio of the amount of substance that has been dissociated from the initial concentration. Thus, it is represented as the fraction of initial to the total concentration.