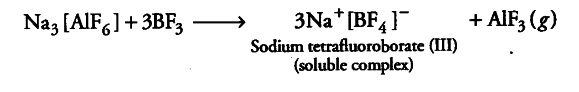Aluminium trifluoride is insoluble in anhydrous HF but dissolves on addition of NaF.
Aluminium trifluoride precipitates out of the resulting solution when gaseous is bubbled through. Give reasons.
(i) Anhydrous HF is a covalent compound and is strongly H-bonded. Therefore, it does not give F ions and hence trifluroaluminate does not dissolve in HF. NaF is an ionic compound. It contains F_ ions which combine with electron deficient trifluroaluminate to form the soluble complex.
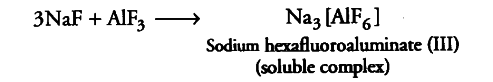
(ii) Boron due to its small size and higher electronegativity has greater tendency to form complexes than aluminium. Hence, precipitation of trifluroaluminate takes place when BF3 is passed through sodiumhexafluroaluminte solution.