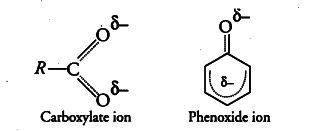
Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Why?
Phenoxide ion has non-equivalent resonance structures in which the negative charge is less effectively delocalised over less electronegative carbon atom and one oxygen atom.
While in carboxylate ion, the negative charge is delocalised over two electronegative oxygen atoms. Thus, carboxylate ion is much more resonance stabilised than phenoxide ion. Since, more stable the conjugate base of an acid, stronger is the acid.