Although geometries of ${ NH }_{ 3 }$ and
$H _{ 2 }O$ molecules are distorted tetrahedral, bond angle in water is less than that of ammonia. Discuss.
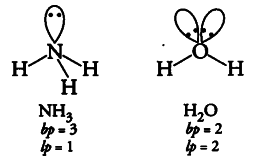
In H _{ 2 }O molecule, there is lone pair-lone pair repulsion due the presence of two lone pairs of electrons while in
{ NH }_{ 4 } molecule there found only lone pair-bond pair repulsion. According to VSEPR theory, the former one is more stronger and hence, the bond angle in water is less than that of ammonia