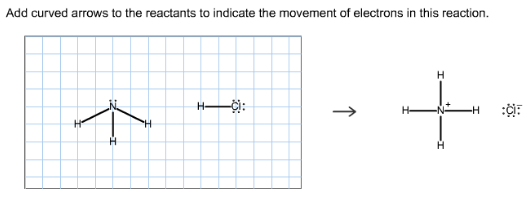
Add curved arrows to the reactants to indicate the movement of electrons in this reaction.
Concepts and reason
Amines react with an acid (HCl) to form protonated amine and halide ion. The lone pair on nitrogen abstracts the proton (hydrogen) from HCl and forms the corresponding products.
The base abstracts the proton from an acid and forms a new bond to the proton of the acid. The acid (HA) loses proton and forms an electron pair on A.
The loss of proton from an acid gives a conjugate base and gain of proton by the base gives a conjugate acid.
Fundamentals
The proton is nothing but the hydrogen atom. In the mechanism, the curved arrow shows the movement of the electron pair. Curved arrow is represented as:

The tail of the arrow begins at an electron pair and the head represents that moving of an electron pair.
Answer:
The reaction is as follows:
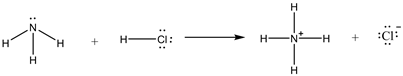
Explanation:
Ammonia is a base, Hcl is an acid. Treatment of ammonia with an acid, HCl, gives ammonium ion and chloride ion. Here, the lone pair on nitrogen abstracts the proton and forms the corresponding products.
The curved arrow representation for the reaction is as follows:
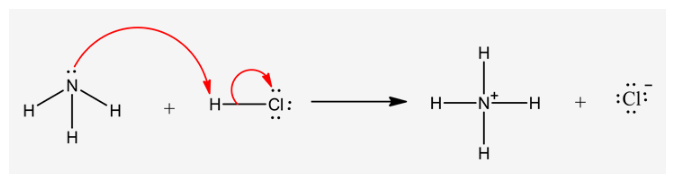
Explanation:
In the curved arrow notation, the tail of the arrow begins from the lone pair of the nitrogen atom in amine and the head points at the hydrogen atom of ![]()
