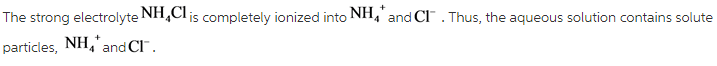Acetone, CH3COCH3 , is a nonelectrolyte; hypochlorous acid,HClO , is a weak electrolyte; and ammonium chloride, NH4Cl , is a strong electrolyte.
Part A
What solute particles are present in an aqueous solution of CH3COCH3 ?
Express your answer as a chemical formula. If there is more than one answer, separate them by a comma. Include charges where necessary.
Part B
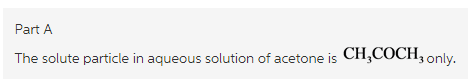
What solute particles are present in an aqueous solution of HClO?
Express your answer as a chemical formula. If there is more than one answer, separate them by a comma. Include charges where necessary.
Part C
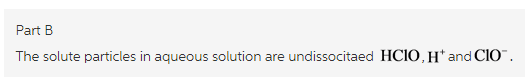
What solute particles are present in an aqueous solution of NH4Cl ?
Express your answer as a chemical formula. If there is more than one answer, separate them by a comma. Include charges where necessary.
Concepts and reason
Electrolyte is a substance that can conduct electricity by dissociation into ions.
Identify the solutes which are weak or strong electrolytes, and observe the ions that produced by solutes.
Fundamentals
Strong electrolyte is a chemical substance that completely dissociated into ions completely.
Chemical substances that partially ionized into ions are called as weak electrolytes.
Answer:
Part A
Acetone is a nonelectrolyte. The substance acetone does not dissociate into ions. The solution of ![]() contains only one solute particle.
contains only one solute particle.
Part B
![]()
Thus, the aqueous solution contains solute particles are undissocitaed ![]()
Part C