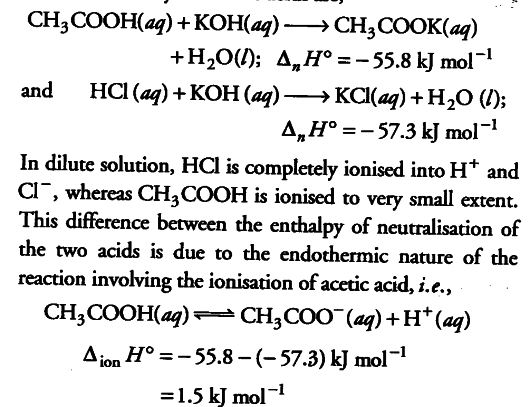Acetic acid (ethanoic acid) and hydrochloric acid react with KOH solution. The enthalpy of neutralisation of ethanoic acid is - 55.8 kj /mol while that of hydrochloric acid is - 57.3 kJ /mol. Can you think of why are these different?
The thermochemical equations for the neutralisation of acetic acid and hydrochloric acids are,