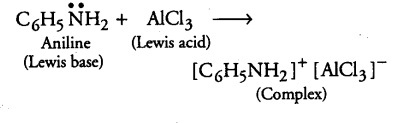Account for the following.
(i) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
(ii) Aniline does not undergo Friedel-OraftsTeaction.
(i) Methylamine being more basic than water, accepts a proton from water and OH- ions are produced which further reacts with ferric ion to give brown ppt. of hydrated ferric oxide.
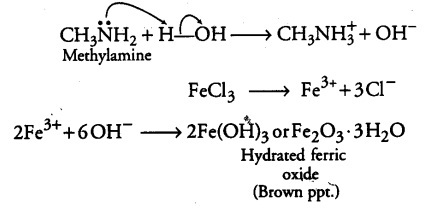
(ii) Aniline being a Lewis base forms a complex with AlC{{l}_{3}}, a Lewis acid. As a result, nitrogen of aniline becomes positively charged and acts as a strong deactivating group for electrophilic substitution reaction. Consequently, aniline does not undergo Friedel-Crafts reaction.