Account for the following observations:
(i) pkb for aniline is more than that for methylamine.
(ii) Methylamine solution in water reacts with ferric chloride solution to give a precipitate of ferric hydroxide.
(iii) Aniline does not undergo Friedel-Crafts reaction
(i) In aniline, the lone pair of electrons on the N atom is delocalised over the benzene ring. As a result, the electron density on the nitrogen atom decreases. In contrast, in CH3NH2, the +I effect of CH3 increases the electron density on the N atom. Therefore, aniline is a weaker base than methylamine. Hence, its pKb value is higher than that of methylamine.
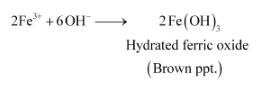
(ii) Being more basic than water, methylamine accepts a proton from water-liberating OH− ions.
![]()
These OH− ions combine with Fe3+ ions present in H2O to form a brown precipitate of hydrated ferric oxide.
![]()
(iii) Being a Lewis base, aniline reacts with Lewis acid AlCl3 to form a salt.
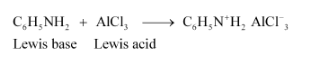
As N of aniline acquires +ve charge, it acts as a strong deactivating group for electrophilic substitution reaction. Consequently, aniline does not undergo Friedel-Crafts reaction.