(a) What is a reactivity series? Describe an activity to develop a reactivity series.
(b) Write two physical and two chemical properties on non-metals
(a) The arrangement of metals in a vertical column in the order of their decreasing reactivities is called Reactivity Series of metals.
Activity:
- Some metals are chemically very reactive whereas, others are less reactive. For example; potassium and sodium react vigorously with cold water so these are very-very reactive metals.
- Calcium reacts with cold water but not vigorously.
- Magnesium does not react with cold water but reacts with hot water.
- Aluminium and zinc react with steam so these metals are less reactive than Magnesium.
- Iron does not react with steam but red hot iron reacts with steam. So, iron is less reactive than aluminium and zinc.
- Whereas, copper and silver do not react with water at all. So, they are very-very less reactive metals. So on the basis of reaction of metals with water, we can arrange
K > Na > Ca > Mg > Al > Zn > Fe > Cu > Ag
(b) Two physical properties of non-metals:
- Non-metals are neither malleable nor ductile.
- Non-metals do not conduct heat and electricity.
Two chemical properties of non-metals:
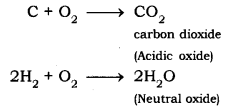
- Reaction of non-metals with oxygen. Non-metals react with oxygen to form acidic oxides or neutral oxides.
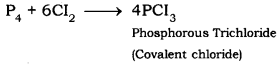
- Reaction of non-metals with chlorine. Non-metals react with chlorine to form covalent chlorides which are non-electrolytes.