(a) The reaction quotient of a reversible reaction is Q. and the equilibrium constant is K .What do you conclude from the reaction if <Kp?
(b)State Le-Chatelier’s principle ?
©In Qualitative analysis, N${{H}{4}}$${{C}{1}}$ is added before adding N${{H}_{4}}$OH solution for testing of III group radicals [${{Fe}^{3+}}$, ${{Cr}^{3+}}$ and ${Al}^{3+}}$]. Explain by using common ion effect ?
(a)If ![]() the reaction tends towards forward j direction to attain equilibrium.
the reaction tends towards forward j direction to attain equilibrium.
(b)Le-Chatelier’s principle :Ifa system in equilibrium is subjected to a change of concentration,temperature or pressure, equilibrium shifts in a direction that tends to undo the effect of the change.
©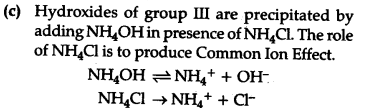
Due to common Ion effect, the degree of dissoriation of N${{H}_{4}}OH is suppressed and less OH- are formed. This less concentration of OH" is suffident to precipitate group HI cations but not the cations of higher groups since the {{K}^{sp}}$of group III < subsequent