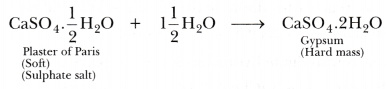A sulphate salt of Group 2 element of the Periodic Table is a white, soft substance, which can be moulded into different shapes by making its dough. When this compound is left in open for some time, it becomes a solid mass and cannot be used for moulding purposes. Identify the sulphate salt and state why does it show such a behaviour. Give the reaction involved.
Answer:
The substance which is used for making different shapes is Plaster of Paris. Its chemical name is calcium sulphate hemihydrate (CaS04.1/2H20). The two formula unit of CaS04 share one molecule of water. As a result, it is soft.
When it is left open for some time, it absorbs moisture from the atmosphere and forms gypsum, which is a hard solid mass.