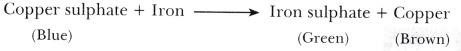
A student took a solution of copper sulphate in a beaker and put a clean iron nail into it and left it for about an hour.
- What changes do you expect?
- Are these changes chemical in nature?
- Write a word equation for the chemical change, if any.
Answer:
-
- Colour of the solution in the beaker changes from blue to green.
2 * A brown coloured deposit is found on the surface of the iron nail.
- Colour of the solution in the beaker changes from blue to green.
- The changes are chemical in nature as new substances, iron sulphate (green) and copper (brown) are formed.