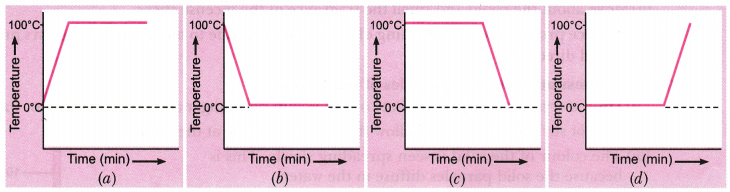
A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following (shown in figure given below) would correctly represent the result? Justify your choice.
Answer:
Since ice and water are in equilibrium, the temperature would be zero. When we heat the mixture, energy supplied is utilised in melting the ice and the temperature does not change till all the ice melts because of latent heat of fusion. On further heating, the temperature of the water would increase. Therefore, the correct option is (d)