(a) State Le-Chatelier’s principle.
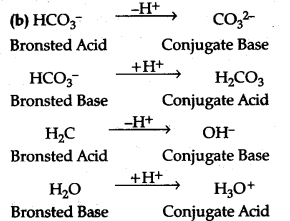
(b) The species H${{Co}^{3-}}$ and ${H}_{2}}$0 can act as both as Bronsted add and Bronsted base. For each case give i corresponding conjugate acid and base.
(a) Le-Chatelier’s Principle : If a system in equilibrium is subjected to change of concentration, pressure or temperature, the equilibrium shifts in the direction that tends to undo the effect of the change.