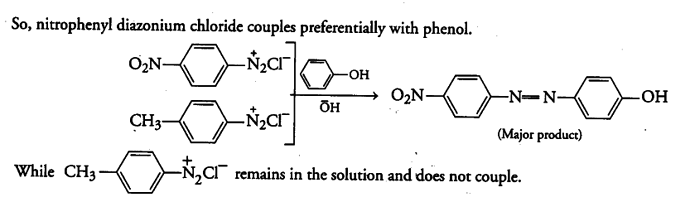
A solution contains 1 g mole each of p-toluene diazonium chloride and p-nitrophenyl diazonium chloride.To this 1 g mole of alkaline solution of phenol is added.Predict the major product.Explain your answer.
The above stated reaction is an example of electrophilic aromatic substitution. In alkaline medium, phenol generates phenoxide. ion which is more electron rich than phenol and more reactive towards electrophilic attack.
The electrophile in this reaction is aryldiazonium cation. As we know, stronger the electrophile faster is the reaction, p-nitrophenyldiazonium cation is a stronger electrophile than p-toluene diazonium cation.