A salt X when dissolved in distilled water gives a clear solution which turns red litmus blue. Explain this phenomenon.
Answer:
Basic solutions turn red litmus paper blue. The salt of a weak acid and a strong base gives a basic solution. So, the given salt X is the salt of a weak acid and a strong base.
Example
When sodium carbonate is dissolved in water, it gets hydrolysed to some extent and forms sodium hydroxide and carbonic acid.
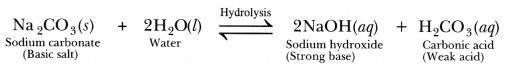
Being a strong base, sodium hydroxide is fully ionised and gives a large amount of hydroxide ions (OH-). Carbonic acid is a weak acid which is only slightly ionised and hence, gives a small amount of hydrogen ions (H+). The H+ ions produced by carbonic acid neutralises only a small amount of OH- ions produced by sodium hydroxide and the rest amount of OH- ions are present in the solution. Hence, the Na2C03 solution is basic in nature. It turns red litmus blue.