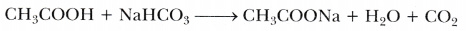A salt X is formed and a gas is evolved when ethanoic acid reacts with sodium hydrogen- carbonate. Name the salt X and the gas evolved. Describe an activity and draw the diagram of the apparatus to prove that the evolved gas is the one which you have named. Also, write chemical equation of the reaction involved.
Answer:
X is sodium ethanoate.
Gas evolved is carbon dioxide.
Activity:
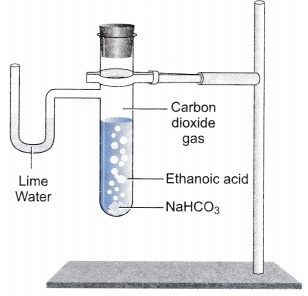
- Set up the apparatus as shown in the figure.
- Take a spatula-full of sodium hydrogencarbonate in a test tube and add 2 mL of dilute ethanoic acid.
- We observe that brisk effervescence of a gas is produced in the test tube.
- Now pass the gas produced through freshly prepared lime water.
- It is observed that lime water turns milky. Only carbon dioxide gas can turn lime water milky. So, this activity proves that when ethanoic acid reacts with sodium hydrogencarbonate, then carbon dioxide is evolved.