a) Rank the following ionic compounds by the magnitude of their lattice energy.
Rank from highest to lowest magnitude of lattice energy.
Highest magnitude to Lowest Magnitude
LiCl, MgO, Na2O, BeO, Na2s
b) Given the following thermodynamic data, calculate the lattice energy of CaBr2(s).
Term Value (kJ/mol) ΔH∘f[CaBr2(s)] -675
ΔH∘f[Ca(g)] 179
ΔH∘f[Br(g)] 112 I1(Ca) 590.
I2(Ca) 1145 E(Br) -325
Express your answer as an integer, and include the appropriate units.
Concepts and reason
The problem is based on the concept of lattice energy. Lattice energy is defined as the amount of energy released when gaseous ions combine to form ionic solids.
Fundamentals
Lattice energy is the energy released when a crystal lattice is formed. It can also be defined as the energy required to break the crystal lattice into gaseous ions. Usually, it is the energy released so it is taken as negative. Lattice energy depends on the distance between the ions and charge on the ions. As the ionic charge increases, the lattice energy also increases and becomes more negative. And as the ions get closer, then also there is an increase in lattice energy.
Answer:
(A)
Lattice energy depends on the distance between ions and charge on ions.
Depending on this condition the order is as follows:
![]()
(B)
Calculate the lattice energy as follows:
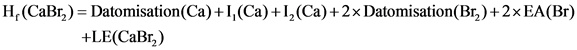
Here,![]() represent the ionization energy, EA represents the electron affinity, LE represents lattice energy.
represent the ionization energy, EA represents the electron affinity, LE represents lattice energy.
Substituting the values in the above equation:

Therefore, the lattice energy of ![]()

