A non-metallic element of group 13, used in making bulletproof vests is extremely hard solid of black colour. It can exist in many allotropic forms and has unusually high melting point. Its trifluoride acts as Lewis acid towards ammonia. The element exhibits maximum covalency of four. .Identify the element and write the reaction of its trifluoride with ammonia. Explain why does the trifluoride act as a Lewis acid?
The only non-metallic element of group 13 is boron. It is an extremely hard black substance and is used in making bulletproof vests. It exists in many allotropic forms and has usually high melting point. Since, B has only s and p-orbitals but no d-orbitals, therefore, at the maximum it can exhibit a covalency of four.
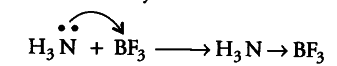
Since, B in BF_{ 3 } has only six electrons in its valence shell, therefore, it needs two more electrons to complete its octet. Thus, BF_{ 3 } acts as a Lewis acid.