(a) Give a chemical test to distinguish between saturated and unsaturated hydrocarbons.
(b) Name the products formed when ethane burns in the air. Write a balanced chemical equation for the reaction showing the types of energies liberated.
(a) Unsaturated hydrocarbons give decoloration of bromin water whereas, saturated hydrocarbon does not decolorise bromine water.
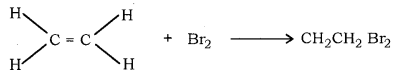
In this reaction, reddish brown colour of bromin is disapperad during the reaction, corifoms the unsatured hydrocarbon.
(b) 2C2H6 + 7O2 → 4CO2 + 6H2O + Heat
Products:
- Carbon dioxide
- water
- Heat.