A dry pellet of a common base B, when kept in open absorbs moisture and turns sticky. The compound is also a by-product of chlor-alkali process. Identify B. What type of reaction occurs when B is treated with an acidic oxide? Write a balanced chemical equation for one such solution.
Dry pellets of sodium hydroxide absorb moisture and turns sticky when kept in open which is also a by-product of chlor-akali process.
Non-metallic oxides are acidic in nature, such as carbon dioxide. When carbon dioxide reacts with sodium hydroxide it gives sodium carbonate and water. Since, sodium hydroxide is a base and solution of carbon dioxide is an acid, so reaction taking place between them is a neutralization reaction.
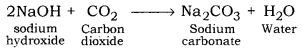
Therefore, common base ‘B’ is sodium hydroxide.
Reaction between ‘B’ and acidic oxide is neutralization reaction.