(a) Distinguish between ionic and covalent compounds in the following properties:
(i) Strength of forces between constituent elements.
(ii) Solubility of compounds in water.
(iii) Electrical conduction in substances.
(b) Explain how the following metals are obtained from their compounds by the reduction process:
(i) Metal M which is in the middle of the reactivity series.
(ii) Metal N which is high up in the reactivity series.
Give one example of each type.
| Properties of distinction |
Ionic compound |
Co-valent compound |
| Strength of forces |
Ionic compound has stronger force of attraction. |
Co-valent compound has weaker force of attraction. |
| solubility |
Ionic compounds are soluble in polar solvent such as; water and insoluble in non polar solvent such as kresone |
Covalent compound dissolves readily in organic solvents such as alcohol and acetone, but is insoluble in polar compound |
| Electrical conduction. |
Ionic compound conducts electricity in its molten state or aqueous form. |
Covalent compounds do not conduct electricity. |
(b) (i) Metals that are present at the centre of the reactivity series are moderately-reactive. Therefore, metal M is a moderately-reactive metal. After obtaining the metal oxides from ores, the metal oxides are reduced to obtain the pure metals. As metal M is a moderately reactive metal, its oxide can be reduced by using coke (carbon).
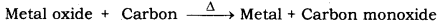
(ii) Metals that are present higher up in the reactivity series are very reactive metals. Hence, metal N is a highly reactive metal. Oxides of highly reactive metals cannot be reduced by using coke. Therefore, these metals are reduced by passing electric current through their molten salts. This process is known as Electrolytic Reduction. One example of metal N : Sodium (Na).