(a) Distinguish between esterification and saponification reactions of organic compounds.
(b) With a labelled diagram, describe an activity to show the formation of an ester.
(a) Esterification reaction is the reaction between carboxylic acids and alcohols. In such reactions, esters get formed. Esters are sweet-smelling organic compounds with the general formula RCOOR. The general reaction for esterification reaction is:
![]()
Alcohol and carboxylic acid can be re-obtained from an ester by treating it with an acid or a base. This reaction is used in the preparation of soaps and is known as Saponification reaction.
![]()
(b) 1 mL of ethanol and lmL of glacial acetic acid are taken in a test tube. 3-4 drops of concentrated sulphuric acid are then added to a test tube. The resulting mixture in a water bath is then heated (as shown in the figure.) for 5 minutes. After this, the contents are poured in a beaker containing 20mL water. A sweet-smelling compound
(ethyl ethanoate) will be obtained as the product.
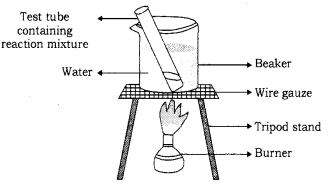
Formation of ester : When ethonoic acid reacts with ethanol in the presence of an acid, ethyl ethanoate is formed. Ethyl ethanoate is an ester and has a sweet smell.
![]()