(a)Differentiate between alkanes and alkenes. Name and draw the structure of one member of each.
(b)Alkanes generally burn with clean flame. Why?
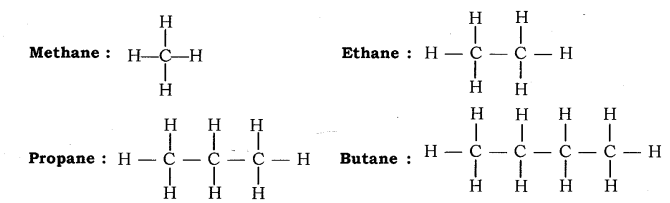
(a) Alkanes: Hydrocarbons in which the carbon atoms are joined by single covalent bond are called Alkanes. They have general formula ![]() where, n is the number of carbon atoms. Suffix, —ane is used while naming alkanes.
where, n is the number of carbon atoms. Suffix, —ane is used while naming alkanes.
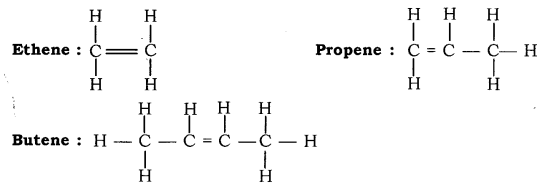
Alkenes: Hydrocarbons in which the carbon atoms are joined by double bonds are called Alkenes. They have general formula CnH2n, where, n is the number of carbon atoms. Suffix, - ene is used while naming allcenes.
(b) Alkanes generally burns with clean flame because in them, the percentage of carbon is comparatively low as compared to other unsaturated hydrocarbons. Hence, they get oxidised completely by the oxygen present in air.