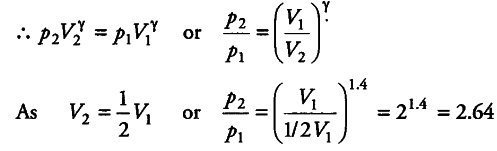A cylinder with a movable piston contains 3 moles of hydrogen at standard temperature and pressure. The walls of the cylinder are made of a heat insulator and the piston is insulated by having a pile of sand on it. By what factor, does the pressure of the gas increase if the gas is compressed to half its original volume?
As no heat is allowed to be exchanged, the process is adiabatic.