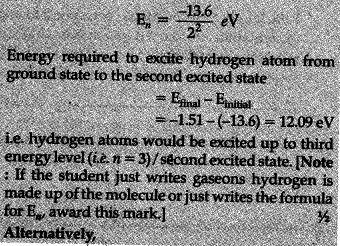A 12.5 eV electron beam is used to bombard gaseous hydrogen at room temperature. Up to which energy level the hydrogen atoms would be excited ?
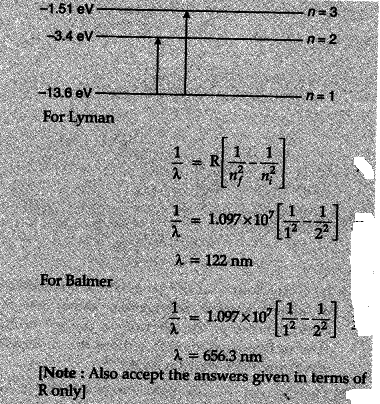
Calculate the wavelengths of the first member of Lyman and first member of Balmer series.
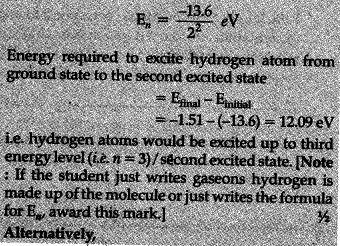
A 12.5 eV electron beam is used to bombard gaseous hydrogen at room temperature. Up to which energy level the hydrogen atoms would be excited ?
Calculate the wavelengths of the first member of Lyman and first member of Balmer series.