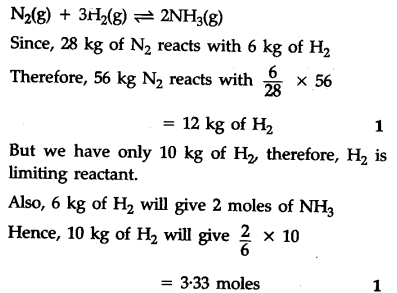56 kg of ${{N}{2}}$(g) and 10 kg of ${{H}{2}}$(g) are mixed to produce N${{H}_{3}}$(g). Calculate the number of moles of ammonia gas formed.
Atomic mass/g ${{m}^{-1}}$ N = 14, H = 1)
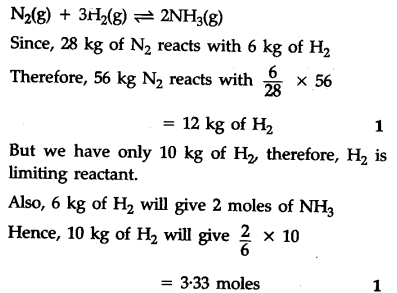
56 kg of ${{N}{2}}$(g) and 10 kg of ${{H}{2}}$(g) are mixed to produce N${{H}_{3}}$(g). Calculate the number of moles of ammonia gas formed.
Atomic mass/g ${{m}^{-1}}$ N = 14, H = 1)