Describe the role of
(i) NaCN in the extraction of gold from gold ore.
(ii) $SiO_{2}$ in the extraction of copper from copper matte. .
(iii) cryolite in the metallurgy of aluminium.
Write the chemical equations for the involved reactions.
(i) Role of NaCN in the extraction of gold is to do the leaching of gold ore in the presence of air from which the gold is obtained later by displacement method.
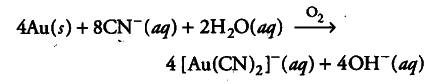
(ii) During smelting and bessemerisation, the impurity, i.e. ferrous sulphide, is oxidised to ferrous oxide which is then reacted with silica (flux) to form slag (ferrous silicate).
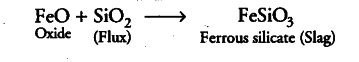
(iii) Melting point of alumina is very high and it is a bad conductor of electricity. So, cryolite is added. Role of cryolite [or fluorspar] are:
(a) It lowers the melting point of the mixture
(b) It also makes a better conductor of electricity.