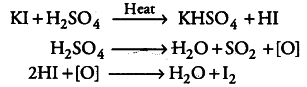
Sulphuric acid is a good oxidising agent. When KI reacts with H_{2}SO_{4}, it produces HI. In order to produce alkyl iodides (R—I) , this HI should react with alcohols (R—OH). But this reaction does not take place in this manner as H_{2}SO_{4} oxidises HI to { I }_{ 2 }, which in turn, does not react with alcohol.
Instead of$H_{2}SO_{4}$, H_{3}PO_{4} can be used to produce alkyl iodides.