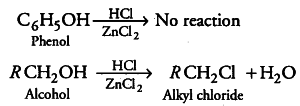
Why can aryl halides not be prepared by the reaction of phenol with HCl in the presence of $ZnC{{l}_{2}}$ ?
Due to resonance in phenol, C— O bond of phenol has some partial double bond character, which strengthens the bond. So, it is difficult to break this C— O bond of phenol while the C— O bond of alcohol is purely single bond and comparatively weaker bond. So, alkyl halides can be prepared by the reaction of alcohols with HCl in the presence of ZnC{{l}_{2}} while aryl halides cannot be prepared by the reaction of phenol with HCl in the presence of ZnCl2.