Which of the following reactions are redox reactions?
Check all that apply.
- Al(s)+3Ag+(aq) = Al3+(aq)+3Ag(s)
- SO3(g)+H2O(l) = H2SO4(aq)
- Ba(s)+Cl2(g) = BaCl2(s)
- Mg(s)+Br2(l) = MgBr2(s)
Concepts and reason
The concept used to solve this problem is based on redox reactions.
A redox reaction is a type of reaction which involves transfer of electrons among two species. This reaction is also called oxidation-reduction reaction.
Fundamentals
In oxidation process, there is an increase in oxidation state of an atom, ion or molecule by losing the electrons.
In reduction process, there is a decrease in oxidation state of an atom, ion or molecule by accepting the electrons.
Answer:
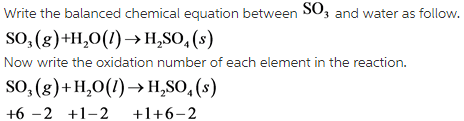
From this reaction, it is clear that there is no change in oxidation number of atoms.
Due to this, this reaction is not a redox reaction.
A redox reaction is a reaction in which there is a change of oxidation number of atoms present in reactant.
Write the balanced chemical equation between aluminum and aqueous solution of silver as follow.
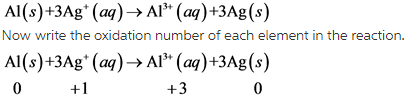
From this reaction, it is clear that Aluminum undergoes oxidation and silver undergoes reduction.
Due to this, this reaction is redox reaction.
In this reaction, oxidation number of Aluminum increases from 0 to +1. So, aluminum undergoes oxidation. The oxidation number of silver decreases from +1 to 0. So, silver undergoes reduction.
Write balanced chemical equation as follow.
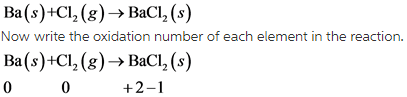
In this reaction, Ba undergoes oxidation and Cl undergoes reduction.
This reaction is redox reaction.
In this reaction, oxidation number of Ba increases from 0 to +2 . So, Ba undergoes oxidation. The oxidation number of Cl decreases from 0 to -1. So, Cl undergoes reduction.
Write balanced chemical equation as follow.
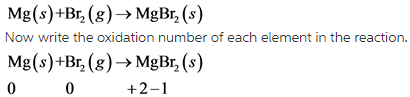
In this reaction, Mg undergoes oxidation and Br undergoes reduction.
This reaction is redox reaction.
The reactions 1, 3 and 4 are redox reactions.
In this reaction, oxidation number of Mg increases from 0 to**+2** . So, Mg undergoes oxidation. The oxidation number of Br decreases from 0 to -1 . So, Br undergoes reduction.