Which an activity to find the change of colour in the reaction of an acid with a base (Neutralization) reaction.
Aim : To test the change of colour in the reaction of an acid with a base.
Required Materials :
- 2 ml of dilute NaOH (Sodium Hydroxide) solution.
- Phenolphthalein indicator solution.
- Dilute HCZ (Hydrochloric) solution.
Procedure : - Take about 2ml of dilute NaOH solution in a test tube.
- Add two drops of phenolphthalein indicator solution.
Observation (i) : - It turns to red or pink colour.
- It shows that NaOH is a base.
Experiment (1) : Add dilute HCZ solution to the above solution drop by drop. Observation (ii) : Pink colour disappears due to the reaction of NaOH (base) with HCZ (acid).
Experiment (2) : Now add one or two drops of NaOH to the above mixture. Observation (iii) : Pink colour reappears on adding NaOH.
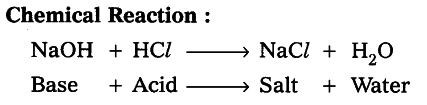
Result: This reaction is called a neutralization reaction.