When solutions of silver nitrate and sodium chloride are mixed, a white precipitate forms. The ionic equation for the reaction is
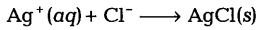
(i) (a) What is the name of the white precipitate?
(b) Is it a soluble or insoluble compound?
(ii) Is the precipitation of silver chloride a redox reaction?
(i) (a) Silver chloride (AgCl) is the white precipitate formed.
(b) Silver chloride (AgCl) is an insoluble compound.
(ii) It is not a redox reaction.
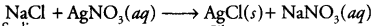
In this reaction, cations Ag+ and Na+ have exchanged their anions NO3- and Cl- and a precipitate of AgCl has been formed. It is an example of double displacement and precipitation reactions.