Part A: What is the net ionic equation of the reaction of FeCl2 with NaOH?
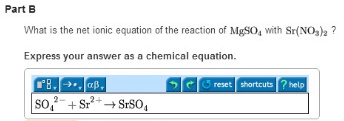
Part B: What is the net ionic equation of the reaction of MgSO4 with Sr(NO3)2?

Concepts and reason
This is based on the concept of Chemical reactions. In many reactions, there are 2 or more ions present in the solution which doesn’t participate in the in the reactions and these ions are called as spectator ions. These ions can be removed from the chemical reaction by describing the chemical reaction.
Fundamentals
A net ionic reaction is the reaction without spectator ions.
Precipitation of insoluble compound occurs from the reactions of ionic compounds. Prediction of spectator ions and precipitation products can be done consulting rules for ionic substance solubility.
These rules are as follows:
Rule 1- Most salts having alkali metal and ammonium cations are soluble.
Answer:
Part A
The total ionic equation is:
Part A
Net ionic reaction is:
![]()
Part B
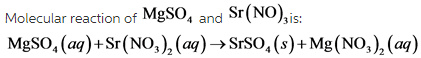
The total ionic equation is:
Part B
The total ionic equation is:
![]()


