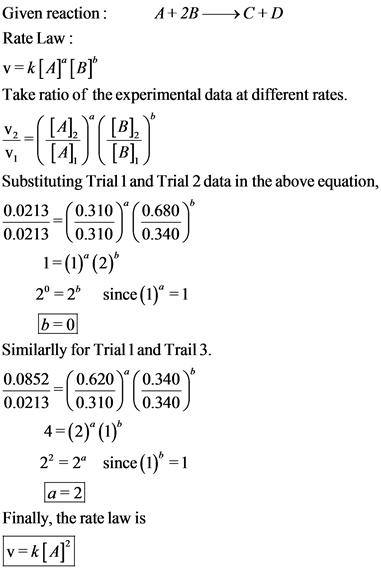
Using the given data, determine the rate constant of this reaction.A + 2B --> C + D
Given Data:
Trial A(M) B(M) RATE (M/s)
1 .310 .340 0.0213
2 .310 .680 0.0213
3 .620 .340 0.0852
k=? the units are M-1 s-1
im pretty sure its Second order because of the units given, other than that im so confused how to find n in order to find the k! Im not getting any whole numbers and when i use the log function i still get the wrong answer, help please! step by step!
Concepts and reason
Rate Law:
In a chemical reaction, the rate law or rate equation is a mathematical expression that relates the rate and concentration of reactants or partial pressures. The rate is directly proportional to the concentration of reactants at aconstant temperature.
The SI unit of rate is M/s.
Rate constant or coefficient:
Proportionality constant links the rate of a chemical reaction to the concentration of reactants at a constant temperature.
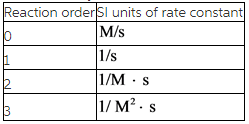
Reaction order:
The order of a reaction is determined by the experimental method.
Step 1: Find the order of each reactant.
Step 2: Find the rate constant using rate equation.
Fundamentals
Rate Equation:
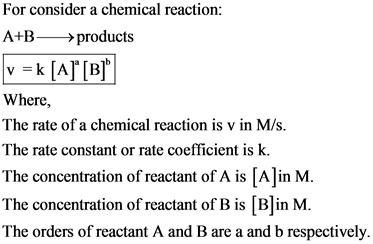
Answer:
The rate equation is used to find the order of all reactants participates in a chemical reaction. Derive the combined rate law taking ratio for two different rates for the same chemical reaction. Plug Trial 1 and Trail 2 data in combined rate equation to find the order of reactant A and Trial 1 and Trial 3 for reactant B. Insert the reactant’s orders in the combined rate law equation to arrive at the final rate equation. The final rate equation is only dependent on the concentration of A.
Do not get confused when substituting the experimental data to find the orders.
Use Trial 1 and Trail 2 data for order of reactant B because of the same concentration of A.
Use Trial 1 and Trail 3 data for order of reactant A because of the same concentration of B.

![]()