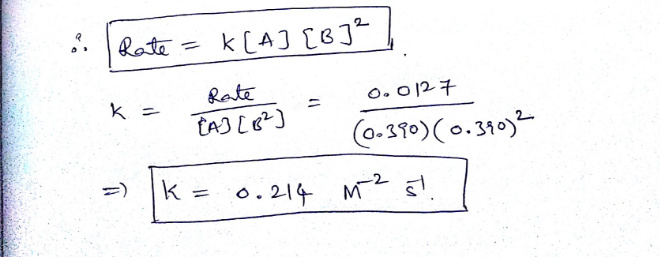
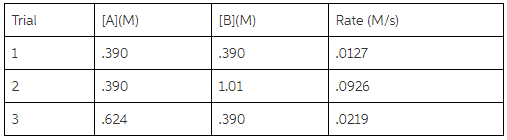
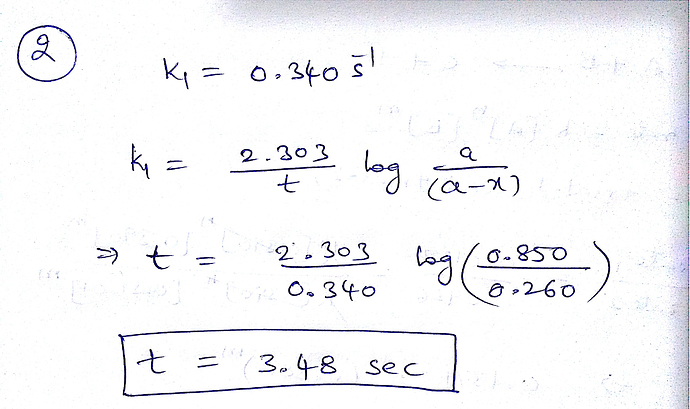1.Using the given data, calculate the rate constant of this reaction.
A+B---------> C+D
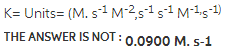
2.The rate constant for this first-order reaction is 0.340 s–1 at 400 °C.
A------> Products
How long (in seconds) would it take for the concentration of A to decrease from 0.850 M to 0.260 M?
Answer is not: 7.85 sec
Answer: