To account for atomic mass of nitrogen as 14.0067, what should be the ratio of 15N and 14N atoms in natural nitrogen ?
[At mass of 14N = 14.00307u and 1SN = 15.001u]
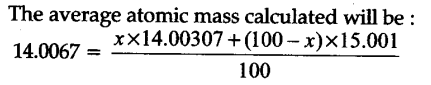
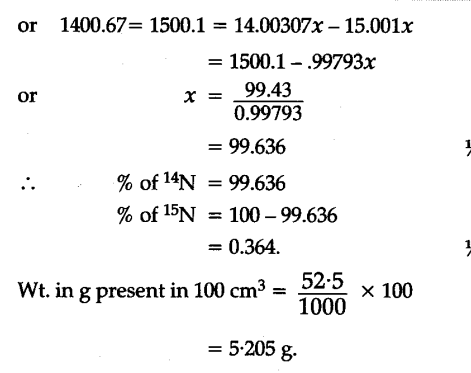
Iska answer glat h.percentage composition ki you baat hi ni hui…100 see divide ni hoga