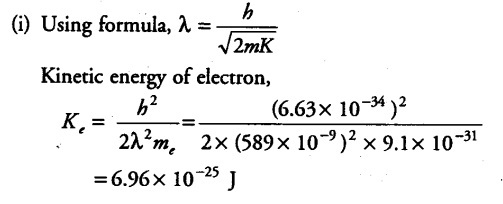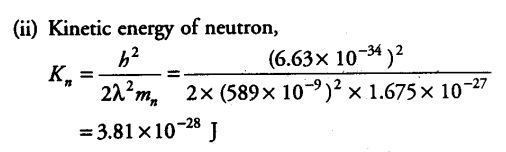The wavelength of light from the spectral emission line of sodium is 589 nm. Find the kinetic energy at which
(i) an electron and
(ii) a neutron would have the same de-Broglie wavelength?
The wavelength of light from the spectral emission line = 589 nm = 589 x {{10}^{-9}} m
Mass of electron, { M }_{ e } = 9.1 x {{10}^{-31}} kg
Mass of neutron, { M }_{ n } = 1.675 x {{10}^{-27}} kg
Planck’s constant , h =6.63 x {{10}^{-34}} J/Kg