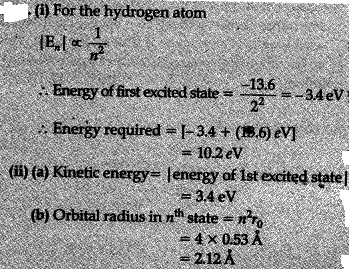The value of ground state energy of hydrogen atom is -13.6 e V.
(i) Find value of required ter move an electron from the ground state to the first excited state of the atom.
(ii) Determine (a) the kinetic energy and (b) orbital radius in the first excited state of the atom. (Given the value of Bohr radius = 0.53 A)