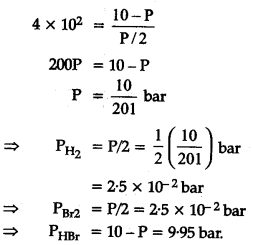The equilibrium constant for the following reaction is 1.6 X 1${{10}_{5}}$ at 1024 K.
![]()
Find the equilibrium pressure of all gases if 10 bar of HBr is introduced into a sealed container at 1024 K ?
Squaring on both sides
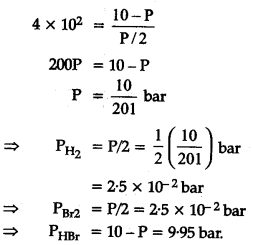
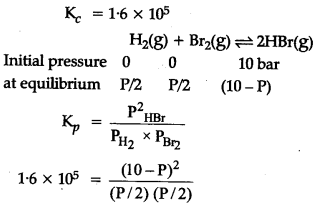
The equilibrium constant for the following reaction is 1.6 X 1${{10}_{5}}$ at 1024 K.
![]()
Find the equilibrium pressure of all gases if 10 bar of HBr is introduced into a sealed container at 1024 K ?

Squaring on both sides