Rank these species by their ability to act as an oxidizing agent.
- Mg2+
- Zn2+
- Cu+
- F2
Concepts and reason
The oxidising tendency of a species is dependent on its reduction potential. When the species has more reduction potential, it means that the specie has more tendency to get reduced and oxidise the other species present in the reaction to behave as an oxidising agent.
More the reduction potential, more the oxidising tendency of the substance.
Fundamentals
Oxidation is defined as the process in which a substance loses electrons and gets oxidised whereas reduction is defined as the process in which the substance accepts electrons and gets reduced.
Oxidising agent is the species that takes part in the chemical reaction, itself gets reduced and oxidises the other species that is present in the reaction.
Reducing agent is the species that takes part in the chemical reaction, itself gets oxidised and reduces the other species that is present in the reaction.
The metals that are present in s block of the periodic table are electropositive atoms which have the tendency to donate electrons and have valence electron that can be lost easily. So, these metals behave as reducing agents.
The non-metals which are present in the p block of the periodic table are electronegative atoms which have the tendency to attract towards themselves. These non-metals behave as oxidising agents.
Answer:
The reduction potential of the given species are as follows:
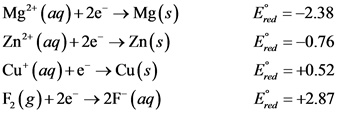
The reduction potential of the given species is as given above.
Negative reduction potential denotes that the species has very less tendency to reduce, it has the tendency to gets oxidised.
Positive reduction potential denotes that the species has high tendency to get reduced and reduce the other specie present in the reaction.
The increasing order of the ability of the species to behave as oxidising agent is as follows:
![]()