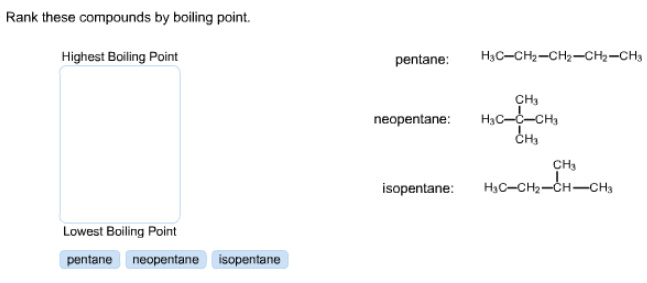
Rank these compounds by boiling point.
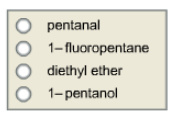
Select the compound that has the highest boiling point, based on that compound’s dominant intermolecular force.
Answer:
highest pentane
lowest neopentane,
1-pentanol has the highest bp among the series because theOH gr in pentanol will form H- bond with another oh gr of adjacent molecule in addition to the attractive LONDON dispersion force between the alkane chain, which is also present in all the cases. but this attractive force is very weak , H-bonding interaction is more stonger, and thus the deciding factor here.