Rank the following compounds in order of decreasing acid strength using periodic trends. HBr, HCl,LiH, H2O from strongest to weakest.
Concepts and reason
According to Arrhenius, an acid is one that donates hydrogen in aqueous solution. That is a proton (hydrogen ion) donor is Arrhenius acid.
The acid, HA donates a proton to base,B forming conjugate acid and base.![]() .
.
Here, HA is acid, B is base.
Fundamentals
Periodic table is composed of 7 periods and 18 groups.
Across a period, the electronegativity of atom determines the acidic strength of the molecule.
Across the period, electronegativity increases. As the electronegativity increases, the atom does not like to share the electrons with proton. Hence, more easy to donate the proton and stronger is the acid. Therefore, across the period acidity will increase.
Down the group, the size of atom determines the acidic strength of the molecule.
Down the group, the size of atom increases. The acid which forms more stable conjugate anion is the stronger acid. The larger the anion is, the more stable the negative charge is stabilized. Therefore, larger the size of the atom, more acidic the molecule is. Hence, down the group acidity increases.
Answer:
Lithium and oxygen belongs to same period and chlorine and bromine belongs to same group.
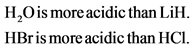
Lithium and oxygen belongs to same period. Hence, the acidity of ![]() is determined using the electronegativity of the atoms other than hydrogen. Electronegativity increases across the period. Therefore, oxygen is more electronegative and lithium. More electronegative the atom is, more acidic the molecule. Hence,
is determined using the electronegativity of the atoms other than hydrogen. Electronegativity increases across the period. Therefore, oxygen is more electronegative and lithium. More electronegative the atom is, more acidic the molecule. Hence,
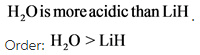
Chlorine and bromine belongs to same group. Hence, the acidity of ![]() is determined using the size of the atoms other than hydrogen. Size increases down the group. Bromine is larger than chlorine.
is determined using the size of the atoms other than hydrogen. Size increases down the group. Bromine is larger than chlorine.
Hence,H - Br bond will be weaker compared to H-cl bond and so, HBr can donate its proton more easily than. Therefore,
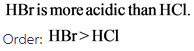
![]()
![]()