Predict the coordination geometries of ${{Ni}^{2+}}$ in which the complex behave as paramagnetic and diamagnetic.
In the given complex, nickel is in +2 oxidation state and the ground state electronic configuration of Ni ion in free gaseous state is

For the given four coordinated complex to be paramagnetic,it must possess unpaired electrons in the valence shell. Tosatisfy this condition, four lone pairs from the four ligands occupies the four ${{sp}^{3}}$hybrid orbitals as :
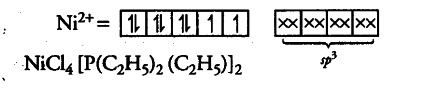
Therefore, geometry of paramagnetic complex must be tetrahedral. On the other hand, for complex to be diamagnetic, there should not be any unpaired electrons in the valence shell. This condition can be fulfilled by pairing electrons of 3d-orbitals against Hund’s rule as:
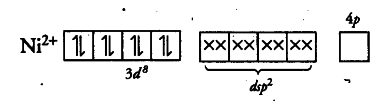
The above electronic arrangement gives d{{sp}^{2}} hybridisation and therefore, square planar geometry.