Nitration of benzene is an electrophilic substitution reaction.
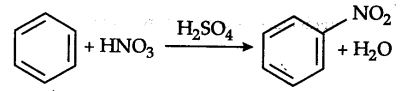
N{{O}^{2+}} (nitronium) ion is the attacking electrophile. In toluene, one H of the ring is substituted by —C${{H}{3}}$group which is an electron releasing group and makes the electrons more readily available to the electrophile, whereas in m-dinitrobenzene, the two —$N{{O}^{2}} groups which are electron-withdrawing in nature makes the availability of electrons of the ring difficult to the electrophile.
Therefore, the ease of nitration decreases in the order toluene>p-{{H}{3}}C--{{C}{6}}$${{H}{4}}$–N{{O}^{2}}>p-${{O}{2}}N--{{C}{6}}$${{H}_{4}}$–N{{O}^{2}}